Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo*; Yoshizuka, Kazuharu*
Proceedings of 21st International Solvent Extraction Conference (ISEC 2017) (Internet), p.131 - 134, 2017/11
Three tridentate extractants including soft and hard donor has been developed and examined. The extractants are termed as 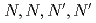 -tetraoctyl-diglycolamide (TODGA), methylimino-
-tetraoctyl-diglycolamide (TODGA), methylimino-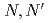 -dioctylacetamide (MIDOA) and
-dioctylacetamide (MIDOA) and 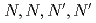 -tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
-tetraoctyl-thiodiglycolamide (TDGA). The results of the present study show that TODGA can extract completely lanthanides and actinides, MIDOA can extract palladium, technetium, and rhenium, and TDGA can extract palladium, silver, and gold. We can compare the distribution ratios of these metals by TODGA, MIDOA, and TDGA. These results can be supported by some spectrometric studies, i.e., IR, NMR and UV, and calculations of metal complexes.
 and Cm
and Cm using a high-performance ligand
using a high-performance ligandSuzuki, Hideya; Tsubata, Yasuhiro; Toigawa, Tomohiro; Matsumura, Tatsuro
no journal, ,
The separation of minor actinide (MA) from HLLW is an important task in the partitioning and transmutation. After MA are separated from HLLW, the mutual separation of Am and Cm
and Cm (Am/Cm separation) can be conducted. A highly practical reagent, called alkyl diamide amine (ADAAM), was developed. ADAAM gives the maximum separation factor up to 5.5. A continuous extraction test was conducted using a multistage countercurrent mixer-settler extractor with ADAAM in n-dodecane. Separation of Am and Cm was demonstrated in very high yield.
(Am/Cm separation) can be conducted. A highly practical reagent, called alkyl diamide amine (ADAAM), was developed. ADAAM gives the maximum separation factor up to 5.5. A continuous extraction test was conducted using a multistage countercurrent mixer-settler extractor with ADAAM in n-dodecane. Separation of Am and Cm was demonstrated in very high yield.
Motokawa, Ryuhei; Endo, Hitoshi
no journal, ,
Sakamoto, Atsushi; Sano, Yuichi; Takeuchi, Masayuki; Watanabe, Masayuki; Koizumi, Kenji
no journal, ,
The effects of O/A ratio on extraction and back extraction performances of uranium in the centrifugal contactor were investigated to apply the same to the co-processing process. The centrifugal contactor was applied to the uranium extraction process with a high stage efficiency in conditions with a wide range of O/A ratios. The back extraction of uranium was achieved at an O/A ratio of less than 2 by the centrifugal contactor. In this condition, the behavior of uranium was evaluated by MIXSET-X with 90 100% stage efficiency.
100% stage efficiency.
Yaita, Tsuyoshi; Suzuki, Shinichi; Kobayashi, Toru; Shiwaku, Hideaki
no journal, ,
Kobayashi, Toru; Suzuki, Shinichi; Shiwaku, Hideaki; Yaita, Tsuyoshi
no journal, ,
The development of separation reagent, which can efficiently separate f-series element such as lanthanide and actinide, is of increasing importance because it concerns establishment of refinement and recycling technique of rare-metals and/or separation procedure in nuclear fuel cycle. Recently, we developed a novel O,N-hetero donor ligand PTA and found that this ligand shows selective coordination with specific trivalent-lanthanide or actinide. In addition, it is revealed that PTA forms two type of complex in structure bordering at recognized lanthanide or actinide. Thus, we investigated the ionic recognition mechanism of PTA based on detailed structural analysis, and proposed novel ionic separation method utilizing the boundary of structural change.
Simonnet, M.; Miyazaki, Yuji; Suzuki, Shinichi; Yaita, Tsuyoshi
no journal, ,
 -radiolysis of a minor actinide extractnt on lanthanides extraction
-radiolysis of a minor actinide extractnt on lanthanides extractionToigawa, Tomohiro; Suzuki, Hideya; Ishii, Sho*; Tsutsui, Nao; Ban, Yasutoshi; Matsumura, Tatsuro
no journal, ,
To evaluate the radiolytic stability of an extractant agents for minor actinides such as N, N, N', N', N", N"-hexaoctyl-nitrilotriacetamide (HONTA), batch extractions of lanthanide (Ln) elements were performed by using  -irradiated extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no initial decrease could be observed.
-irradiated extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no initial decrease could be observed.
Ueda, Yuki; Morisada, Shintaro*; Kawakita, Hidetaka*; Omi, Kentaro*; Fujita, Mitsuharu*; Weigand, J.*; Shimojo, Kojiro; Naganawa, Hirochika; Oto, Keisuke*
no journal, ,
Platinum group metals (PGMs) are indispensable metals in modern industries and are used for example as active components in automobile gas catalysts, jewelry goods, electronic devices and dental materials. In order to meet the growing demand, high efficient extraction reagents for liquid-liquid extraction of the respective anionic chloro-complexes are required in order to enhance capable winning from either pour geological deposits or complex secondary sources within the Urban Mining. The ligand containing urea or amide groups can form the hydrogen bond with anionic species. The novel urea or amide incorporating extraction reagents were synthesized and its extraction behavior and mechanism were investigated in this work.
Shimojo, Kojiro; Okamura, Hiroyuki; Imura, Hisanori*; Naganawa, Hirochika
no journal, ,
The detection and removal of toxic metal ions have received considerable attention from the viewpoint of environmental protection. Therefore, it should be highly desirable to develop new analytical methods that can simultaneously detect and remove toxic metal ions. In this study, the solvent extraction of toxic metal Cd(II) cations was investigated using a novel extractant composed of diaza-18-crown-6 and two 8-quinolinol chromophores. When using ionic liquid (IL) or chloroform as extracting medium, the intramolecular cooperative effect of this ligand for Cd(II) cations was observed only in the IL systems, resulting in dramatic enhancement of the extraction performance of this ligand in IL compared with that in chloroform. Furthermore, it was found that this ligand became luminous by forming the extracted complexes with Cd(II) cations.
Suzuki, Shinichi; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
no journal, ,
Sugita, Tsuyoshi; Fujiwara, Iori*; Okamura, Hiroyuki; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
The extractant plays a key role in solvent extraction. Previous studies reveal that acidic extractant with tridentate structure, DGAA, has superior extraction performance for various metal ions. in this study, to investigate a effect of each functional group in DGAA-type extractant, we carried out are synthesis and evaluation of analogous extractants of DGAA. In the result, the secondary amide-type DGAA tended to provide better extraction for relatively small-sized metal ions than tertiary amide-type DGAA, and the thioether chain-type DGAA showed superior extraction ability for soft metal ions compared to ether chain-type DGAA.
Okamura, Hiroyuki; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
no journal, ,
Ionic liquids have been widely investigated as novel functional extraction media in solvent extraction. We have investigated the synergistic ionic-liquid extraction of lanthanoids(III) (Ln(III)), and revealed that the synergistic effect is effective for the selective separation of Ln(III). In this study, the synergistic ionic-liquid extraction of Ln(III) with 2-thenoyltrifluoroacetone and trioctylphosphine oxide in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was investigated to evaluate the synergistic effect. In the ionic liquid system, the synergistic effect on selective extraction for heavier Ln(III) was observed in contrast to the conventional organic solvent systems. The slope analysis indicated that the neutral adduct and the cationic ternary complexes are extracted. The adduct formation constant of the cationic ternary complex increased with an increase in the atomic number. These results indicate that the formation of hydrophobic charged ternary complexes is a main factor for the selective synergistic effect toward heavier Ln(III).
Eguchi, Ayano; Morita, Kotaro*; Okamura, Hiroyuki; Hirayama, Naoki*
no journal, ,
In chelate extraction of metal ions into organic solvents, formation of their uncharged complexes with ligands is required. Moreover, coordinatively saturated (unhydrated) complexes are more preferable as extracted species. On the other hand, in ionic liquid chelate extraction, ionic liquid can extract charged species and coordinatively unsaturated (hydrated) species. In this study, the effects of bidentate ligands on ionic liquid chelate extraction of trivalent metal ions, Fe(III), Al(III), Ga(III) and In(III), into 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C mim][Tf
mim][Tf N], n=2,4,8) was studied using 8-quinolinol (HQ) and 2-mercaptopyridine
N], n=2,4,8) was studied using 8-quinolinol (HQ) and 2-mercaptopyridine  -oxide (HSPyO) as extractants. The extraction selectivity order for trivalent metal ions was Fe
-oxide (HSPyO) as extractants. The extraction selectivity order for trivalent metal ions was Fe  Ga
Ga  In
In  Al for HQ and Fe
Al for HQ and Fe  In
In  Ga
Ga 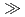 Al for HSPyO. Namely, HSPyO was favorable for extraction of In(III). The extractability of Ga(III) was in the order of [C
Al for HSPyO. Namely, HSPyO was favorable for extraction of In(III). The extractability of Ga(III) was in the order of [C mim][Tf
mim][Tf N]
N]  [C
[C mim][Tf
mim][Tf N]
N]  [C
[C mim][Tf
mim][Tf N]
N]  chloroform for HSPyO, whereas reversed order was obtained for HQ. These results were seemed to be based on difference in concentration of deprotonated bidentate ligands in water.
chloroform for HSPyO, whereas reversed order was obtained for HQ. These results were seemed to be based on difference in concentration of deprotonated bidentate ligands in water.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; et al.
no journal, ,
PUREX process was established for industrial scale reprocessing plant. TRUEX and the 4 group separation were developed for partitioning of minor actinides from high level liquid waste from reprocessing process, and demonstrated by the continuous extraction test using genuine high level liquid waste. Although the extractants for reprocessing and MA separation processes, such as tri-n-butyl phosphate (TBP), n-octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide (CMPO) and diisodecylphosphoric acid (DIDPA), have excellent performance for recovery of U, Pu or MA, the molecules contain phosphorus which could be cause for the secondary waste from the solvent extraction processes. To minimize the radioactive waste from nuclear fuel cycle, we have conducted research and development of the new reprocessing and MA separation processes using innovative extractants in accord with CHON principle.
Naganawa, Hirochika; Nagano, Tetsushi; Yanase, Nobuyuki*
no journal, ,
A new liquid-liquid extraction apparatus, named, emulsion-flow extractor, where low cost, simplicity, compactness, high processing speed, high efficiency, and safety go together, is introduced. The emulsion-flow technique can actualize very efficient liquid-liquid extraction with its high two-phase mixing ability to an emulsion by spraying micrometer-sized oil droplets into a counter-current aqueous solution. Meanwhile, this technology can also actualize more than tenfold processing speed in its very rapid phase separation and less than one fifth cost with its very simple structure workable by only solution sending in comparison with mixer-settler that is the most popular industrial liquid-liquid extraction technique. In addition, the emulsion-flow extractor having no drive part in its main body has less trouble and is safer than the conventional extractor.